Introduction
If you want to stop relying on subjective impressions and start measuring whether a peptide serum, prebiotic scalp treatment or home device is actually changing your hair, converting scalp photos into density scores is one of the most practical at‑home approaches. This detailed guide walks you through hair biology basics, exact photo protocols, image processing workflows, statistical analysis, troubleshooting, and how to present results — so you can confidently compare products and draw useful conclusions.
Why quantify hair density at home?
- Objectivity: Photos turned into numbers reduce bias versus memory or visual impressions.
- Repeatability: A standardized method enables direct before/after comparison for the same person.
- Decision support: You can compare peptide serums, prebiotic scalp treatments, and devices on the same scale and stop the guesswork.
- Documentation: If you’re testing multiple products over months, a numeric log helps you identify what truly moved the needle.
Quick overview of the process
- Set up consistent photo capture (lighting, scale, angle, sampling sites).
- Calibrate scale in each image so pixels map to real dimensions.
- Crop a fixed region of interest (ROI) for counting (commonly 1 cm²).
- Preprocess (grayscale, blur, contrast) and threshold to separate hairs from scalp.
- Count hairs or follicles manually or automatically.
- Compute hairs per cm² and summary metrics like terminal:vellus ratio.
- Log data, apply simple statistics, and visualize change over time.
Brief hair biology primer (what we measure and why it matters)
- Hair density: the number of hairs per unit area (commonly hairs/cm²). Clinically meaningful and directly measurable from surface images.
- Follicular units: groups of 1–4 hairs from the same follicular opening — counting follicles vs hairs can give different insights.
- Terminal vs vellus hairs: terminal hairs are thicker and pigmented; vellus are thin and lighter. Treatments may convert vellus to terminal hairs.
- Hair cycle: anagen (growth), catagen (transitional), telogen (resting). Visible density changes lag behind molecular effects because of cycle timing — expect measurable results typically after 8–24 weeks depending on the intervention.
What you’ll need (detailed kit & alternatives)
- Camera options
- Smartphone with macro lens attachment (cheap, portable)
- USB digital microscope (20–200x) — best clarity for follicle-level detail
- Compact camera with macro mode
- Lighting
- LED ring light for diffuse, shadow‑free illumination
- Softbox or bright indirect daylight as alternatives
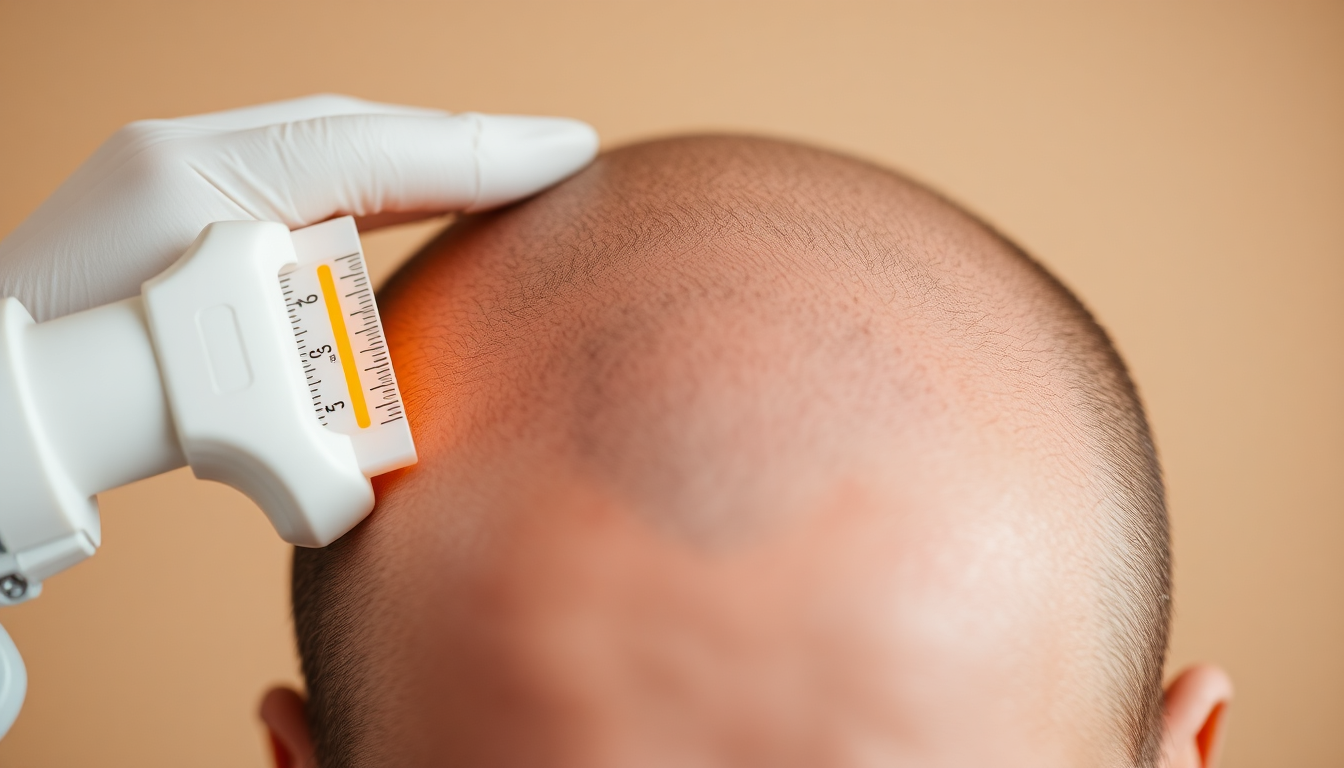
- Scale reference
- Millimeter ruler sticker or printed calibration card stuck near the ROI
- Common coin (remember to use the same coin each time and measure its diameter in mm)
- Stabilization: tripod, phone mount, or flexible arm to keep angle and distance constant
- Hair clips, small skin marker or temporary dot to identify sampling sites
- Image analysis software: ImageJ/Fiji (free), smartphone measurement apps, or Python/OpenCV for automation
- Spreadsheet or database to log all measurements and metadata
- Optional: microscopic calibration slide if using a microscope
Choosing sampling sites and marking them
- Pick reproducible anatomical landmarks: vertex (crown), mid‑scalp, frontal hairline. Avoid extremely curved areas where scale alignment changes.
- Mark spots using temporary dots on the scalp margin (near hair part) or create a fixed parting with clips. Photograph marks every session to verify location consistency.
- If comparing left vs right (split‑scalp) use mirrored marks and randomize which side receives which treatment when possible.
Photo setup: step‑by‑step for consistent images
- Clean the scalp the same way before imaging — ideally 24–48 hours after last wash so natural oil levels are comparable.
- Set your lighting: position the ring light to provide even illumination without specular highlights. For phones, lower exposure slightly to avoid blown highlights.
- Place scale object in the same plane as the scalp and visible within the frame. If using a ruler sticker, place the sticker adjacent to the ROI.
- Use the same camera distance each session. Measure distance once and mark a spacer (e.g., a small foam spacer of set height) to reproduce it.
- Stabilize the camera and ensure it is perpendicular to the scalp. Use a spirit level app if necessary to maintain angle consistency.
- Take at least three images of each sampling site per session, slightly varying the framing to avoid missing data due to reflections or hair strands in one image.
- Name files with date_site_repeat (e.g., 2025-08-01_vertex_r1.jpg) and back them up to a private folder.

Camera settings & practical tips
- Use manual focus or tap to focus on the scalp in macro mode — autofocus can hunt and blur micro details.
- Set ISO low to reduce noise; raise ISO only if lighting is insufficient.
- Use RAW capture if available to allow more flexible postprocessing.
- Avoid flash directly on the scalp; use continuous LED lighting for evenness.
- If using a USB microscope, ensure the software’s resolution and scale calibration are saved consistently across sessions.
Calibration: mapping pixels to mm (critical for comparability)
Calibration must be present in each image. Two common approaches:
- Ruler/sticker method: include a millimeter‑precise sticker or small ruler in the same plane as the ROI. Use the sticker to set pixels/mm in ImageJ or other software.
- Coin method: if you use a coin, measure its true diameter and set that as the calibration distance when visible. Keep the same coin in every session.
In ImageJ: Analyze → Set Scale → Draw a line over the known-length object → Enter Known Distance (mm) and unit (mm). ImageJ will then convert pixel measurements to mm and cm².
Selecting the region of interest (ROI)
- Choose a fixed physical area. A 1 cm² ROI is common because the density value converts directly to hairs/cm².
- When using rectangular pixel ROIs, compute area using calibration and crop identical physical sizes each time.
- Avoid areas with scars, large growths, or obviously atypical features unless you intend to measure those specifically.
- Document ROI coordinates relative to anatomical landmarks so anyone repeating your method can match them.
Image preprocessing — preparing images for counting
- Convert to grayscale to simplify processing.
- Apply a gentle Gaussian blur (radius 1–2 pixels depending on resolution) to reduce sensor noise while preserving hair edges.
- Increase local contrast using histogram equalization or brightness/contrast adjustments so hairs appear darker vs scalp.
- If hair colors are light, you may need to invert the image or use color channel separation to enhance contrast.
Thresholding and segmentation
Thresholding separates hair pixels (foreground) from scalp (background). Options:
- Global thresholding: pick an intensity cutoff. Works if lighting is uniform.
- Adaptive thresholding: computes local thresholds across the image — better for uneven lighting.
- Otsu’s method: an automated global thresholding technique useful as a starting point.
After thresholding you may need morphological operations (erosion, dilation) to remove small speckles and join broken hair pixels into continuous structures.
Counting hairs: manual, semi‑automated, and fully automated methods
- Manual counting
- Open the ROI and mark each hair root as a point. ImageJ’s Cell Counter plugin is ideal for this.
- Pros: accurate when done carefully; cons: time consuming and subject to human error/fatigue.
- Semi‑automated counting
- Use thresholding and Analyze Particles in ImageJ. Tune particle size parameters to exclude noise. You may still need to manually correct false positives/negatives.
- Pros: faster; cons: requires parameter tuning and manual QC.
- Automated counting with computer vision / machine learning
- Skeletonize hair structures and count endpoints, or use connected components to identify hair roots.
- Deep learning models (U‑Net, Mask R‑CNN) can be trained to segment follicles and discriminate vellus vs terminal hairs but require labeled training data.
- Pros: scalable for many images; cons: development overhead and labeled data needed.
Dealing with overlapping hairs, hair direction, and broken hairs
- Overlapping hairs: thresholding may join overlapping strands. Skeletonization followed by endpoint counting can help locate roots rather than full strands.
- Hair direction: avoid counting mid‑shaft intersections as roots. Focus on where hairs emerge from the scalp (visible follicular openings if present).
- Broken or very short vellus hairs: set a minimal hair length or diameter rule for inclusion and apply consistently across all timepoints.
Measuring additional metrics: diameter, follicular units, and coverage
- Hair diameter: If your imaging magnification and calibration are sufficient, measure hair thickness by sampling cross‑sections or measuring hair width in pixels and converting to mm.
- Follicular unit density: count visible follicular openings per cm² if you want follicle‑level metrics rather than hair counts.
- Coverage percentage: estimate the fraction of the ROI not covered by scalp skin (use thresholding to compute foreground pixel area vs total area).
- Terminal:vellus ratio: if you can separate hairs by diameter or intensity, report the proportion of terminal hairs — a sensitive marker of treatment converting vellus to terminal hairs.
Computing a density score and related summary numbers
- Hairs/cm² = (hair count in ROI) / (ROI area in cm²). If ROI = 1 cm², count = density directly.
- Follicles/cm² = (follicular unit count) / (ROI area).
- Coverage (%) = (foreground pixel area / total pixel area) × 100.
- Mean hair diameter (mm) = average measured widths across sampled hairs.
- Composite density score: you can create a weighted index combining hairs/cm², terminal ratio, and coverage to summarize overall change (document weighting scheme transparently).
Example ImageJ (Fiji) workflow with tips
- Open image > Image > Set Scale (use ruler/coin)
- Crop using a fixed cm² ROI (Plugins or rectangle tool with size in mm after calibration)
- Image > Type > 8‑bit
- Process > Filters > Gaussian Blur (radius 1)
- Enhance Contrast > Equalize Histogram
- Image > Adjust > Threshold — choose method (Otsu) and apply
- Process > Binary > Open / Close to clean
- Analyze > Analyze Particles — set size (in mm^2 range) to exclude specks and record count
- Validate: overlay counted particles on original image and manually verify a subset
Sample Python/OpenCV script (expanded pseudocode)
import cv2
import numpy as np
img = cv2.imread('roi.jpg')
gray = cv2.cvtColor(img, cv2.COLOR_BGR2GRAY)
# calibrate using known pixel/mm ratio saved per image
pixel_per_mm = get_calibration(img) # user-defined
# crop to ROI by pixel coords based on calibration
roi = gray[y1:y2, x1:x2]
# denoise and equalize
blur = cv2.GaussianBlur(roi, (5,5), 0)
eq = cv2.equalizeHist(blur)
# adaptive thresholding
th = cv2.adaptiveThreshold(eq,255,cv2.ADAPTIVE_THRESH_GAUSSIAN_C, cv2.THRESH_BINARY_INV,11,2)
# morphological clean
kernel = cv2.getStructuringElement(cv2.MORPH_RECT,(3,3))
clean = cv2.morphologyEx(th, cv2.MORPH_OPEN, kernel, iterations=1)
# find connected components
num_labels, labels, stats, centroids = cv2.connectedComponentsWithStats(clean)
# filter by area to remove tiny noise
hair_candidates = [s for s in stats[1:] if s[cv2.CC_STAT_AREA] >= min_area_pixels]
hairs_per_cm2 = len(hair_candidates) / roi_area_cm2
print('Estimated hairs/cm^2:', hairs_per_cm2)
Logging data: spreadsheet template & metadata to record
Columns to include:
- Date
- Subject ID (if more than one person)
- Sampling site (e.g., vertex, left frontal)
- Product/device used and regimen (dose, frequency)
- Time since last wash
- Image filenames (raw and ROI)
- ROI area (cm²)
- Hair count
- Hairs/cm²
- Terminal:vellus ratio (if available)
- Notes (lighting issues, missed measurements)
Statistical analysis for single‑person and small N experiments
- Single‑person repeated measures: report mean and standard deviation of multiple ROIs per session. Compute percentage change from baseline and report confidence intervals where possible.
- Paired tests: if comparing baseline vs post‑treatment for the same sites, a paired t‑test (parametric) or Wilcoxon signed‑rank test (nonparametric) can be used to assess significance. Ensure assumptions (normality) are checked.
- Multiple sites/subjects: analyze with mixed‑effects models or repeated‑measures ANOVA to account for within‑subject correlation.
- Power and sample size: for clinical‑like studies, small effect sizes require dozens of subjects. For personal self‑experiments, focus on effect magnitude and repeatability rather than formal p‑values.
How to visualize and present your findings
- Time series plots: hairs/cm² on the y‑axis, time (weeks) on the x‑axis, with separate lines for different products or sites.
- Boxplots: show distribution across ROIs or subjects at baseline and endpoint.
- Bar charts for percentage change with error bars for standard error or confidence intervals.
- Overlay example images: before and after ROIs side by side with annotated hair counts for qualitative context.
- Include metadata table with regimen and imaging conditions so others can interpret your data.
Practical study protocol templates
Two templates you can adapt:
- 12‑week single product trial
- Baseline: capture 3 images per site after 24–48 hours post‑wash
- Apply peptide serum per manufacturer instructions daily
- Re‑image every 4 weeks (weeks 4, 8, 12) with the same protocol
- Analyze and report hairs/cm², terminal ratio, and coverage
- Split‑scalp comparative trial (peptide serum vs prebiotic scalp treatment)
- Randomize sides. Baseline imaging as above.
- Apply Product A to left side, Product B to right side for 24 weeks.
- Image every 8 weeks and maintain regimen log.
- Statistical analysis: paired tests comparing left vs right within subjects.
Common pitfalls and troubleshooting
- Inconsistent scale: always include a scale object; without it, cross‑session comparisons are unreliable.
- Lighting changes: if brightness or color temperature changes, thresholding will fail. Use fixed lighting or normalize intensity during preprocessing.
- Hair styling or product residue: styling products can make hairs clump or shine. Photograph at consistent intervals after washing.
- Counting errors: validate automated counts by manually reviewing a random subset of images to estimate false positives/negatives.
- Small ROI bias: sample multiple ROIs per site to reduce local variability and better reflect average density.
Privacy, consent, and data storage
- Personal images of the scalp are personally identifying medical data for some people. Keep images in a secure folder with access controls.
- If sharing case studies publicly, blur faces and obtain written consent from the subject.
- Document metadata but avoid storing unnecessary personal identifiers. Use anonymized IDs if you plan to publish results.
How this compares to professional trichoscopy
- Professional trichoscopy uses dermatoscopes and standardized devices and may measure miniaturization and perifollicular changes more precisely.
- At‑home methods are less precise but can identify meaningful relative changes if the imaging protocol is strictly standardized.
- For diagnostic or severe cases consult a dermatologist rather than relying solely on an at‑home measurement protocol.
Case study example (walkthrough with numbers)
Participant A runs a 24‑week split‑scalp trial of a peptide serum vs a prebiotic scalp treatment. Baseline (week 0)
- Left (peptide): 110 hairs/cm²
- Right (prebiotic): 108 hairs/cm²
Week 12
- Left: 125 hairs/cm² (+13.6%)
- Right: 118 hairs/cm² (+9.3%)
Week 24
- Left: 138 hairs/cm² (+25.5% vs baseline)
- Right: 128 hairs/cm² (+18.5% vs baseline)
Interpretation: Both sides improved, but the peptide serum side showed a larger absolute and relative increase. Statistical significance would require multiple subjects or repeated ROI variability analysis; in a single subject this suggests the peptide regimen may have had greater effect but is not definitive.
Frequently asked questions (FAQ)
- Q: How soon will I see changes?
A: Visible density changes usually appear after 8–12 weeks for topical serums, and 12–24 weeks for device‑driven approaches. Individual response times vary. - Q: Can I automate everything?
A: Many steps can be automated but accurate segmentation for hair root detection often needs manual checks or trained ML models. - Q: Is this medical advice?
A: No. This is a methodology guide. For medical concerns or severe hair loss consult a dermatologist.
Tools, resources & further reading
- Fiji / ImageJ: https://imagej.net/Fiji — free image analysis platform with a large plugin ecosystem.
- OpenCV documentation & tutorials for Python: https://opencv.org/
- Sample macros and plugins: search ImageJ community forums for hair/fiber counting plugins.
- Academic literature on hair density measurement (search PubMed for trichoscopy and hair density studies).
Illustrations and example images
The images embedded in this article illustrate the photo setup, a raw cropped ROI, a thresholded binary mask, and an overlay showing counted hair points. Alt attributes include keywords to improve accessibility and SEO:
Recommended products and where to start testing
If you want to test proven cosmetic routines alongside your measurement workflow, consider integrating an evidence‑driven peptide serum and prebiotic scalp treatment into your regimen. For example, explore the range of targeted options and devices available at Eelhoe hair growth products. Specific items that pair well with photographic monitoring include:
- Peptide serums — lightweight serums absorb quickly and are easy to standardize in a trial.
- Prebiotic scalp treatments — formulated to support scalp microbiome balance and scalp barrier health.
- Eelhoe devices — home devices that pair with topical regimens; if you test a device, document session frequency and duration carefully.
Ethical note on product links
Links above are provided as examples of the types of products that fit well with the methodology outlined here. If you plan to run a split‑scalp or N‑of‑1 trial, track products, batch numbers, and application amounts precisely.
Final checklist before you start a trial
- Decide on sampling sites and mark them permanently for the trial duration.
- Create a regimented imaging schedule and stick to it.
- Prepare a logging spreadsheet with all metadata fields populated.
- Run a pilot session, process images, and verify your counting workflow produces reproducible numbers across 3 repeated photos.
- Set realistic expectations: small short‑term fluctuations are normal; look for consistent trends over weeks to months.
Conclusion: measure to learn, then purchase with confidence
Turning scalp photos into density scores transforms subjective impressions into actionable data. With careful imaging, calibration, and consistent analysis, you can compare peptide serums, prebiotic scalp treatments and home devices on the same objective scale and iterate toward a routine that works for you.
If you’re ready to begin a structured trial and want a curated set of products to test, consider browsing Eelhoe hair growth products. Their targeted peptide serums, prebiotic scalp treatments, and compatible Eelhoe devices are designed to integrate smoothly into a photographic monitoring workflow. Consider adding a product from Eelhoe to your trial and use the methods in this guide to quantify real change — if you like what you see, you’ll have the data to support continued use and future purchases.
Appendix A: quick troubleshooting guide
- Blurry images — increase shutter speed or stabilize camera; ensure focus is on scalp.
- Overexposed highlights — reduce exposure or diffuse the light source.
- Too much noise — lower ISO and improve lighting; use mild denoising in software.
- Threshold fails — try adaptive thresholding or preprocess with histogram equalization.
- Counts disagree with visual impression — validate automated counts against manual counts for a subset.
Appendix B: glossary
- Anagen — growth phase of hair
- Vellus hair — fine, short, non‑pigmented hair
- Terminal hair — thick, pigmented hair
- ROI — region of interest
- Thresholding — image segmentation into foreground and background
Appendix C: useful links and references
- ImageJ / Fiji: https://imagej.net/Fiji
- OpenCV: https://opencv.org/
- Eelhoe product catalog: https://eelhoe-cosmetics.com (sponsored)
Good luck with your at‑home hair density experiments. With patience, a careful protocol, and consistent documentation, you’ll be able to tell whether a peptide serum, prebiotic scalp treatment or device is genuinely helping your hair — and if you want a ready set of products to include, check Eelhoe for options designed to fit trials like this.







Leave a comment
All comments are moderated before being published.
Situs ini dilindungi oleh hCaptcha dan berlaku Kebijakan Privasi serta Ketentuan Layanan hCaptcha.